Kw and the autoprotolysis of water.
In an aqueous environment there are incredible amounts of water molecules that continuously collide with each other.
The water molecule has the (very weak) tendency to donate protons, but also to take protons (so water is amphoteric)
During collisions of two water molecules, the following can happen:
H2O |
+ |
H2O |
 |
H3O+ |
+ |
OH- |
|
|
ΔH > 0 |
| weak base |
|
weak acid |
|
strong acid |
|
strong base |
|
|
|
Att.: the two ions are produced in equal amounts.
The water equilibrium only can be realised in aqueous environment, and is dislocated very much to one side, and has an equilibrium constant:
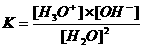 →
→
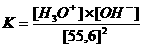 →
→
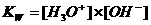
In neutral aqueous solutions with temperature of 25ºC the following will happen:
Of each mol water molecules (about 0,6 x 1023) "only" 6 x 1016 water molecules will effectively catch a proton
and the same number of 6 x 1016 water molecules will donate.
In water of 25ºC the concentration [H3O+] = [OH-] = 10-7 mol/dm3 or: pH = pOH = 7
Kw (autoprotolysis constant) = K x (55.6)2 = [H3O+] x [OH-] = 10-14 mol2/l2
That autoprotolysis of water is an endothermic process.
At high temperatures more ions are produced, their concentrations increase (for example: 10-6 mol/dm3 instead of 10-7 mol/dm3).
The value of Kw will change in this case from 10-14 to 10-12.