The nucleus of the atom
Atoms have a nucleus, which is built up of nucleones (nuclear particles, the most important ones: protons and neutrons.
The nucleus has its position in the middle of the atom.
The nucleon (one nuclear particle) has a mass of 1 a.m.u. (more about this later). All nucleons, nuclear particles toghether determine the atomic mass.
The presence of protons provides the nucleus with a positive charge.
Where neutrons are neutral, every atom nucleus must have a postive charge.
The (positive) nucleus attrackts the (negative) elektrons.
Changes in the nucleus are called: 'nuclear reactions'.
Protons & neutrons
Protons & neutrons (the nucleons) are the biggest particles in the nucleus. In the nuclear physics have been discovered many more particles.
f.e. the nuclear particles are built up of quarks. More information can be found on the internet
(link).
Those small particles stay out of this course.
Protons and neutrons have about the same mass, but different charge: protons are positive and neutrons are neutral.
The positive protons attract the negative electronshells around the atom. Ehe neutrons take care for the stability in the nucleus. That is needed because equally charged particles to repell (the positive protons).
The very short distance forces of masses appear toe be stronger thant the repelling electric forces.
The proportion between the number of protons and neutrons in the nucleus is extremely important for the needed stability.
Radioactivity & radiation
A wrong proportion between the number of protons and neutrons will cause an unstable nucleus. Such an unstabel nucleus has the tendency to look for more stability.
That can be found, only if in the nucleus the proportion between protons and neutrons will change. There must be added neutrons, or the number of protons must change.
In unstable nuclei such a process is natural, spontaneous. It just goes on. You can't do anything against it.
Often the nucleus will reach stability via a series of this kind of changes. they can take milliseconds, but sometimes also millions of years.
This type of changes (that the nucleus is managing alone) we call that: 'natural radioactivity'. They are natural nuclear reactions.
It can go differently:There are artificial ways for nuclear reactions, for example if an atom nucleus is being shot with certain very energy rich particles. The nuclei then are made unstable in an artificial way. You've got 'artificial radioactivity'.
This whole phenomena is called radioactivity.
The steps that an atom can take to find more stability, thus to change itself, are limited to the following two options:
- One neutron can change ointo a proton. An electron is made, and the atom will lose that electron and send it away with lots of energy (=β-radiation).
This will happen when an atom has too many neutrons.
- The second option for an unstable atom is to send away from the nucleus packets of four nucleons, out of the nucleus, out of the atom even.
Such a packet is composed of 2 protons and 2 neutrons; and it has a positive charge. We call this packet a α-particle or α-radiation.
This process has the preference if there are not enough neutrons in the nucleus.
When something changes in the nucleus, you call that a nuclear reaction. You can write such a reaction in a chemical way, with a so called nuclear reaction equation.
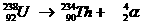
This is a natural nuclear reaction, whereby the atom nucleus of an Uranium aton sends away a packet of 4 nuclear particles.
A new atom is born: Thorium.
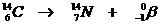
This is a natural nuclear reaction, whereby a radioactive Carbon atom has too many neutrons, and therefor starts to change one of them into a proton.
En electron is made and powerfully sent away.
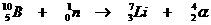
This is an artifical nuclear reaction, whereby a neutron with great power was shot inside the nucleus of a Borion atom.
That's how the atom becomes unstable and suffers a nuclear reaction: a packet of four nuclear particles is immediately removed, as a reaction to the attack.
Fuel for nuclear plants can be Uranium(235) of which the nuclei catch a neutron, and then start a nuclear reaction that is extremely exothermic (creates lots of energy).
The other isotope, Uranium(238) can also catch a neutron and form Plutonium(239).
In its turn, this Plutonium can serve as basic material in the production of atom bombs.