addition?
A carbon chain can have double or triple bonds between the C-atoms. In that case the substance is called: 'unsaturated'. This means, the double bonds can open and absorp C-atoms at both open ends.
Such a bonding opens (in one way or another) and the C-atoms at both sides have an open spot, an unpaired electron
Another suitable atom can come and fill that open place.
Attention: we do not talk here about substitution where an atom is substituted, but about addition where a new chemical bond is made with such a C-atom.
Imagine: two people love each other and hold both hands. Then they lose one hand and connect to the hand of someone els.
So addition is the reaction between an unsaturated substance that saturates itself with new connected atoms or atom groups.
We can approach this phenomena addition with the so called π- and σ-bonds.
These bonds exist in one molecule if the carbon chain has double or tripel bonds.
We can say: there is an overlap of hybride-orbitals of the sp2 and/or sp type. In both bonding types σ (linear overlap) and π (parallel overlap). Only the π-bonds participate in the addition.
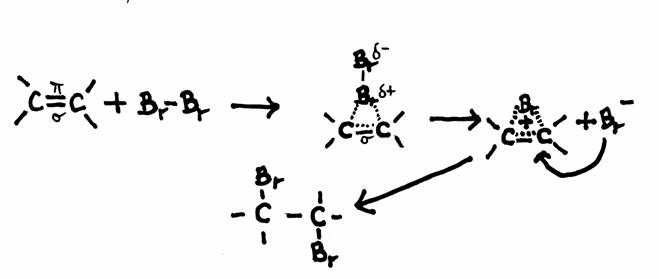
Example of a possible mechanism for addition: Bromium and ethene
Ethene has a double bond, so a place with a higher electron density (four electrons) and thus with a higher concentration of negative charge.
That negative charge is causes particularly by the π-electrons.
If a Bromine molecule approaches this bond, kind of polar induction is created in the Bromine molecule and something like an 'addition bridge' is formed.
Another Bromine atom kan now approache this bridge from the other side, as we see in de drawing.
Alkenes can suffer addition with various substances, like:
Bromine and the other halogenes; Hydrogen; Water; and more.
Att: mostly a catalyst is needed for a good addition reaction.
Calciumcarbide (C2H2) is a white and solid substance with a special smell, very unstable, that spontaneously reacts with water.
The products of this reaction with water are: a gas with a sharp smell and a basic solution. If the gas is guided through Bromine water (a diluted solution of Bromine in water), the yellow colour slowly disappears.
The ethyne gas (acetylene gas) that is produced in this reaction, is a very energy rich substance.
In the old days, it was applied in lamps/ torches. Today we use it in particular in welding.
