cracking crude oil
The most important components of crude oil are carbonhydrogens with relatively long chains (the quality of the cruede oil depends on that).
The various carbonhydrogens can be separated by means of distillation, but there is a problem:
Most molecules are too big and therefore a big part remains behind as tar and afphalt; and we get too few applicable products.
Look at the traffic: you need a lot of asphalt, but much more petrol and diesel (having much smaller molecules) to drive on that asphalt.
To improve the amount of smaller molecules in crude oil, there is a method of 'cracking':
Strong heating of the crude oil (please no oxygen present!) can break those big molecules in smaller ones.
We are talking about main processes (cracking and then distillation) of the oil refineries.
Specially the cracking process needs very good catalysts.
The final products of the oil refinery, those with the smaller molecules, in there turn, are raw materials for the chemical industry and often build up of the elements Hydrogen and Carbon: the Carbonhydrogens.
You might name them: carbonhydrides.
They are the raw materials for many derivates of the chemical industry, applied for society, as: petrol, plastic, nylon, etc.
Information: wikipedia
A distillation tower:
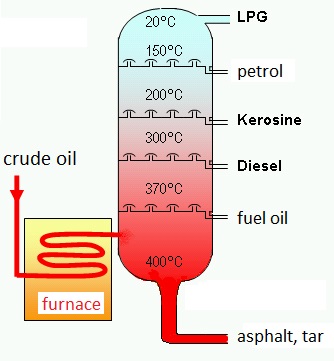
Fractions with a high boiling point will condense low in the tower where the temperature is very high; fractions with a low boiling point will only condense in the upper regions of the tower.
Crude oil will be separated in various components (fractions)with increasing molecular weight. This kind of distillation is sometimes called 'fractionation'.
At the same time impurities in the crude oil are removed. The upcoming products can serve as fuel or as basic raw material for many petrochemical product.
The flowdiagramme of a modern refinery:
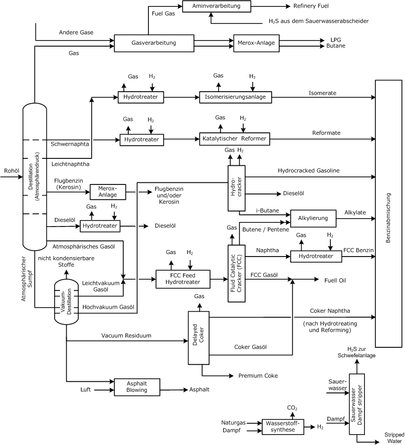
An oil-refinery is an installation, a plant for the refinery of crude oil into usefull products.
History
The very basic oil plant was created by the American Samuel Kier in 1855. It was nothing more than a vessel wherein the content was tried to distill in the same way as done by the alcohol industry.
We talk about a batch process whereby the energetic yield was neglected. The aim was: production of kerosine, to be used as lamp oil (petroleum).
The lighter fractions, as petrol, were considered as damaging and dangerous. The residu was not further fractionated, but sold als fuel (gasoil).
In Europe however there was more attention for efficiency. crude oil was found a lot and easily, but useful fuel was scarce and expensive.
In Galicia, in 1871, for the first time a system was applicated with two vessels upon each other: in the lower one the residu was distilled for a second time and the heavyestfractions (bitumen) were separated en removed. Peruts made another step in the direction of a continuous process and only once in four days the process needed to be restarted, to remove the bitumen in the lower vessel.
In 1875,Fuhst, developped a system composed of a number of vessels, connected in series, with in between a system of overlope pipes. The residu of the one vessel flowed into the next one. Also the coolers were connected in series, in order to use the cooling water as efficient as possible. The fractions have, as they get more heavy, a higher outflow temperature; their boiling points are higher.
The aim of all this was to avoid redistillation (rectification). This was needed because the products of the primitive refineries were composed of too many different fractions.
The first complete continuous refinery process started in Bakoe, in 1873, but custom authorities did not trust the case, fearing tax defaulting.
in 1880 - 1881 Alfred Nobel built an installation, a plant, consisting of 17 vessels, capable to work continuously. He asked patent for it. This technology was now going to dissimilate in het whole world. Shell was one of the first.
Soon were distinguished nine groups of product:
- Refinery gases (propane, butane, pentane)
- Petrol (America: gas)
- Kerosine or petroleum
- lubricants
- Grease
- Mineral oil
- Paraffina
- Bitumen
- Fuel oil
For a long time, kerosine was the most important product, but a number of invents (such as combustion motor) would change the whole pattern.
From 1920 onwards was developped the petrochemistry, and a number of light products (named: naphta) got a useful destination.
Further developments
An important characteristic of the refinery is its flexibility. Meaning: the refinery is capable to refine several kinds of crude oil, even if they are very different, and also to deliver the needed products in the right amounts.
The composition of crude oil is well known, and difficult to change; but the refinery must be capable to influence the fractions, through mixing processes.
In this process, cracking is extremely important, with help of catalysts (catalitic cracking) and with Hydrgen (hydrocracking). The heavier hydrocarbons can be cracked into lighter ones. In this way, it is possible, for example, to increase the yield of petrol.
These processes were already developped at the end of the 19th century, but processes of catalytic reforming (with Platinum as catalyst) were introduced only after 1940. This because of the needs during the second world war. Naphta was converted in more complex compounds, such as aromatic compounds and branched chains (isomerisation).
This not only produced petrol with a high octane number, but also basic materials for the petrochemical industry.
Side processes in the refinery can be: desulphurisation and hydrogen plants.
Distillation of crude oil
Every component in crude oil has its own boiling point. Therefor the crude oil is heated up to 350-370°C where the oil changes from liquid to gaseous in the distillation tower. The vapours are rising in the distillation tower and simultaneously cooled. The most heavy components have higher boiling points, the lighter ones have lower boiling points. The heavy ones will condensate first, the lighter ones will rise more.
At the end different products can be separated at various hights, levels of the tower.
| fraction |
temperature |
C atoms
per molecule |
| gas |
<20°C |
1-4 |
| light naphta |
20-80°C |
5-6 |
| heavy naphta |
80-175°C |
7-10 |
| kerosine |
175-260°C |
10-14 |
| gasoil |
220-350°C |
9-25 |
| residue |
>350°C |
>25 |
Hydro-treating
Difficult atoms, causing problems during the process like S, N and O can be removed from the fractions through reactions with Hydrogen, whereby bonds as C-S, C-N and C-O are broken.
The aim of hydrotreating is:
- protecting catalysts from being poisoned
- improvement of properties
- protection of the environment
Gasoil in particular contains a lot of sulphur. Without removal of the sulphur, air pollution is the result; SO2 creates acid rain.
Sulphur also must be removed, making the products as Naphta unapplicable for further treatment.
Sulphur finds itself in so called thiol-groups. Adding Hydrogen and a catalyst can take the sulphur out of the thiols and change it into hydrogensulphide. The last can be changed into elementary Sulphur of into gypsum, plaster.
Catalytic Reforming
With help of a Pt-catalyst, chains can be branched or changed into cyclic hydrocarbons.
Cracking
Mostly the conversion of heavier into lighter fractions.
The lighter fractions could be gases too. But these gases are converted into petrol, if possible.
Polymerisation
Under influence of a catalyst, two or more olefinemolecules are connected. The result is a mixture of isomers containing only one double bond. Normally they have a higher octane number than homologes of paraffine.
Alkylation
Alkylation is a reaction between olefines and isobutane to create strongly branched alkanes. The meaning of this is the production of petrol with a high octane number. It starts with low molecular alkenes and isobutane.


