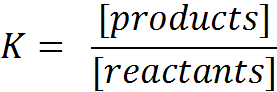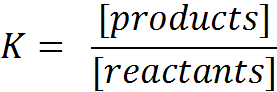KC and KP
The equilibrium constant contains the concentrations of the participating homogeneous substances. We add a C: KC.
The other homogeneous situation is in the case of a gas-equilibrium. We do not exactly talk about concentrations, but more about (partial) gas pressure of every participating gas. Every gaseous component in a gas contributes to the pressure.
The partial pressure of a component is proportional with the amount of that gas.
In the formula of the equilibrium constant we fill in the values of those partial pressures; the formule does not really change, but the K is sometimes indicated with KP.
resuming:
- In homogeneous solutions we use KC
- In gases we use KP
- In heterogeneous equilibira of (s)+(aq) we use Ks
- Agreement exist about the 'concentration' of a solid, a heterogeneous substance: it has value 1.
- The value of K does not depend on changes of concentrations, pressure or catalyst.
Changes only depend on temperature change of the equilibrium environment.
There are many equilibria with extreme strong substances at one side an very weak ones at the other side of the arrows.
Again: if equilibrium was reached, de concenctrations at both sides can differ enormously. Almost certainly there will be a lot of the weak and very little of the strong substances.
Such an equilibrium is dislocated, shifted very much to one side.
You can notice that immediately, looking at the formula for K and the value of K: